On April 25, in order to further strengthen the quality supervision of epidemic prevention materials and standardize the export order, the Announcement No.12 (2020) of the Ministry of Commerce, the General Administration of Customs, and the State Administration for Market Regulation on Further Enhancing Quality Oversight for Exported Epidemic Prevention and Control Supplies was issued. It indicates that the customs shall check and release the goods on the basis of the Name List of Medical Devices and Supplies Companies with Certification/Authorization from other Countries
The SARS-CoV-2 IgM/IgG Antibody Test (Colloidal Gold) produced by JOINSTSAR was selected into the white list of such products for export. Also we have CE certification, and we are applying for the registration of the FDA and ANVISA for Brazil.
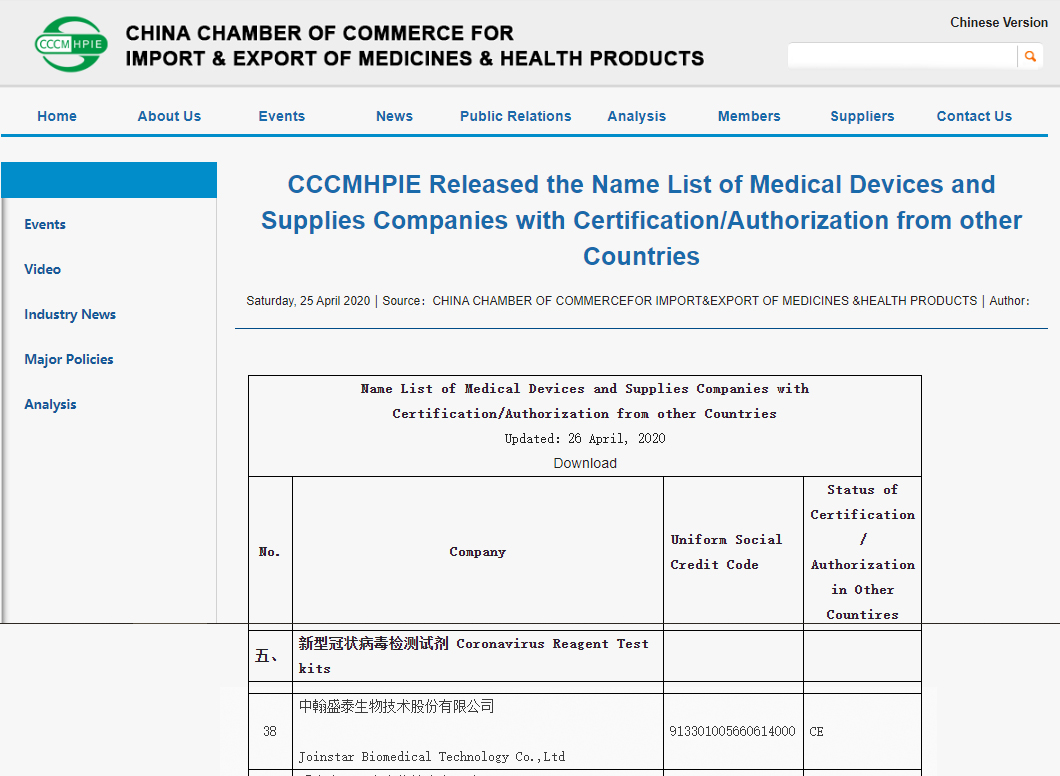
At the time of the outbreak of the pandemic, JOINSATR, as always, adhere to the concept of "focus on medical diagnosis, serve human health", strictly control the export quality, and take practical actions to help people from all over the world to fight against the COVID-19 and safeguard life and health, so as to establish Chinese brand on the international stage.

SARS-CoV-2 IgM/IgG Antibody Test (Colloidal Gold)


Above: CE DOC & ISO





